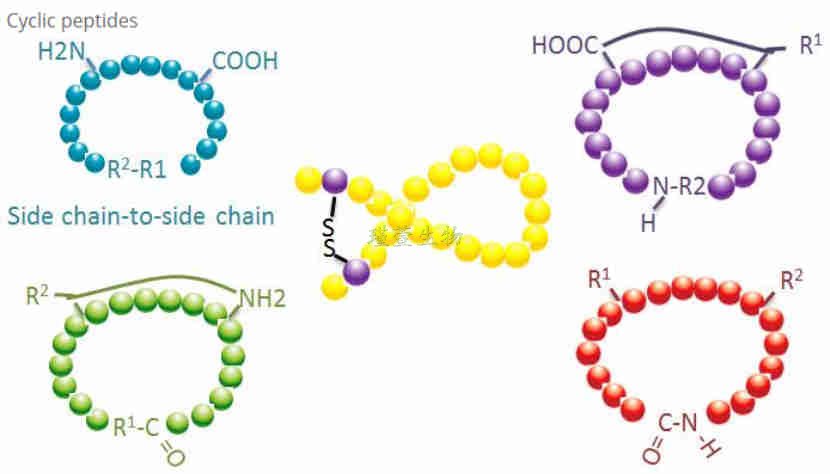
Cyclic peptides have many applications in biomedicine and many natural peptides with biological activity are cyclic peptides. Because cyclic peptides tend to be more rigid than linear peptides, they are extremely resistant to the digestive system, can survive in the digestive tract, and exhibit a greater affinity for target receptors. Cyclization is the most direct way to synthesize cyclic peptides, especially for peptides with the large structural framework. According to the way of cyclization, it can be divided into the side chain-side chain, terminal-side chain and terminal-terminal (head-tail connected).
(Picture source:Boston Cellron)
Our Services:
Head-tail cyclization
A ring is formed by condensation of N-terminal amino and C-terminal carboxyl groups of the polypeptide. We ensure that this reaction has a very high yield, usually 98% or more purity.
Side chain ring formation
Gxene Biotech can achieve the exact site of polypeptide loop formation by selective protection and removal of the amino group of the Lys or Orn side chain and the carboxyl group of the Glu or Asp side chain.
Disulfide bond ring formation
Disulfide-bonded cyclic peptides have stable chemical structures and can resist high temperature and enzyme attack. Therefore, the research and development of disulfide cyclic peptide have great market potential. Our company has matured technology for the synthesis of one pair of disulfide bonds, two pairs of disulfide bonds and multiple pairs of disulfide bonds.
Stapled Peptides
Hydrocarbon stapled peptides, also known as stapled peptides, are a cyclic method of a polypeptide that introduces chemical crosslinking at specific sites, locking miniproteins into their biologically active α -helix conformation. Their bookbinding peptides are highly bioactive and stable in quality.

 Sweep WeChat yards pay attention to us
Sweep WeChat yards pay attention to us
