Gxene Biotech has a mature antibody production platform with well-established antibody engineering technology to ensure the quality of service. Gxene Biotech has extensive experience in antibody library construction and supported by our technology and platform, Gxene Biotech can provide high-quality human Fab antibody library services. Gxene Biotech is constantly upgrading its technology to better serve your needs. With many years of research and development, Gxene Biotech has established a solid platform for Fab library construction. Our scientists can offer high-quality phage display library construction and custom phage display library screening services to precisely meet our clients' demands.
Human Fab Antibody Libraries:
The basis for antibody recombination is the ability to obtain sequence information from monoclonal hybridoma antibodies. The Fab fragment is the main region for antibody recombination. The Fab fragment, which is the antigen binding region, determines an antibody's specificity and affinity. It consists of one variable region (V), and one constant area (C1) for each heavy and lighter chain of the antibody.
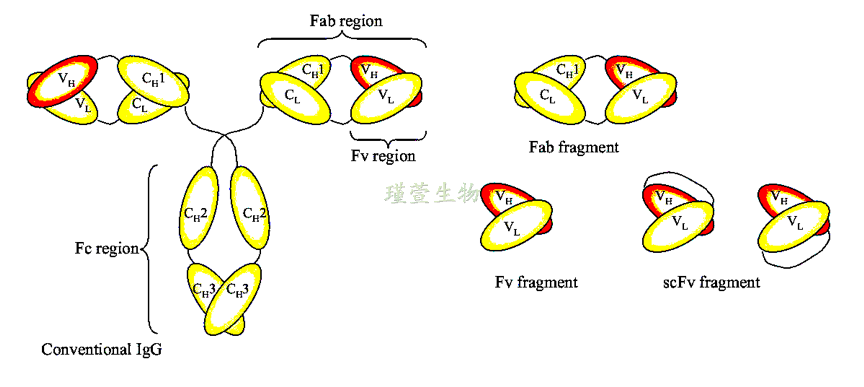
The Fab antibody library refers to VH and VL, especially the CDR region genes of the antibody form a large number of different clones by different strategies, such as amino acid mutations and frameshift mutations. In addition, Gxene Biotech can provide a suitable solution: after sensitizing human peripheral blood monocytes in vitro, high-affinity monocytes were isolated by flow cytometry, and then EBV monoclonalization was conducted. Using this method, highly consistent monocyte clones can be obtained, then carry out library construction.
Types of Phage Display System:
Gxene Biotech has extensive experience in the construction of phage display libraries. Our phage display platform is well-established and we have been able to use it for various types of phages. The M13 phage platform is the most widely used for phage display. It is also extensively used in many research areas. There are a variety of phage display services available via T4 and T7 phage system, which may offer different promising options depending on their particular merits.
Service Process:
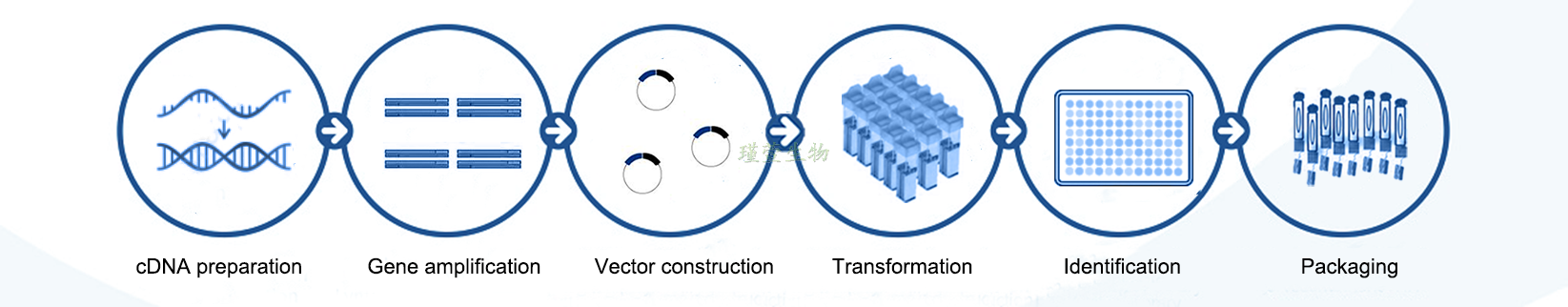 Service Procedure:
Service Procedure:
|
Steps |
Specification |
Timeline |
Deliverables |
|
Monoclonalization of peripheral blood mononuclear cells |
* Blood mononuclear cell acquisition.
* Monocyte sensitization.
* Monoclonalization.
* Monoclonal culture after flow cytometry. |
8-12 weeks |
* Experimental report: including detailed construction procedures and representative sequence information.
* Product delivery: including bacterial and phage antibody libraries. |
|
cDNA synthesis (Clients can provide cells) |
* Total RNA extraction.
* RT-PCR.
* PCR amplification with Actin-specific primers to identify the quality of cDNA. |
|
Library construction |
* Primer design & synthesis
* PCR amplification of variable region genes of heavy and light chains using cDNA as a template.
* scFv gene splicing.
* Plasmid construction & transformation: after enzyme digestion, scFv and phagemid vector were ligated and transformed into TG1 host bacteria by an electric shock to construct antibody libraries.
* Identification: 20-50 clones were randomly selected, PCR identification, sequencing and analysis of antibody sequences.
* Packaging: >1×1013 phage particles /ml. |
Service Highlights:
--Extensive experience in antibody engineering development
--Guaranteed high affinity up to10-7-10-13
--High throughput expression analysis
--High capacity with unique clone count up to 107 - 1012
--Detailed sequence analysis
--Strict quality assurance: cGMP management rules
--Multiple phage display systems: M13, T4, T7
--One-stop services, including antibody development, antibody sequencing, antibody labeling and related services


 Sweep WeChat yards pay attention to us
Sweep WeChat yards pay attention to us
