


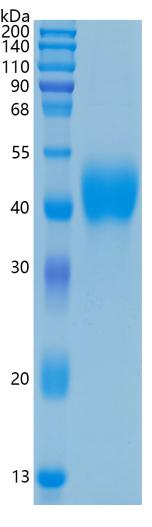
研究方向
TFPI 基因编码一种 Kunitz 型丝氨酸蛋白酶抑制剂,对于调节凝血过程中的组织因子 (TF) 依赖性途径至关重要。该途径由因子 VIIa-TF 复合物的形成启动,激活其他蛋白酶(因子 IX 和 X)并最终形成纤维蛋白凝块。TFPI 蛋白充当自动调节环抑制剂,针对激活的 X 因子和 VIIa-TF 蛋白酶。在血友病动物模型中,抑制这种蛋白质有望恢复止血。该基因产生多种蛋白质亚型,每种亚型的抑制活性、特异性和细胞定位都不同。值得注意的是,TFPI 在多种组织中表现出广泛的表达,包括肝脏、胎盘等。
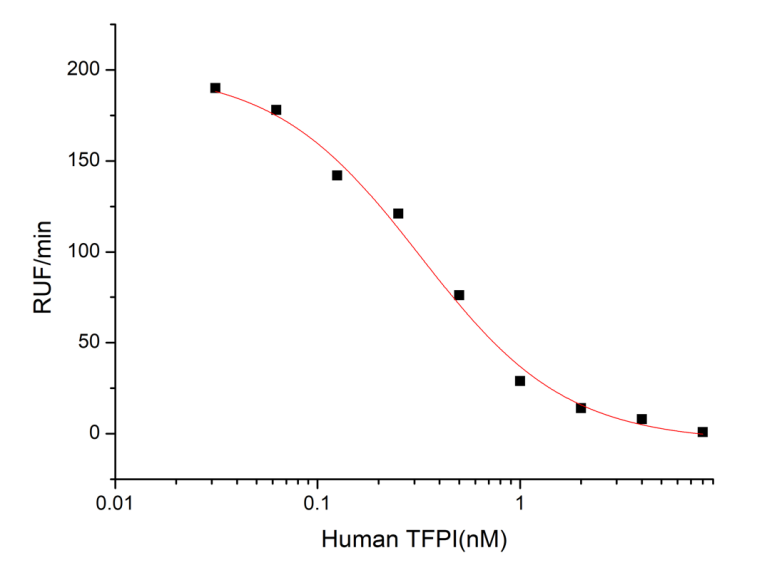
Measured by its ability to inhibit trypsin cleavage of a fluorogenic peptide substrate, Mca-RPKPVE-Nval-WRK(Dnp)-NH2. The IC50 value is <0.41 nM.
Tissue factor pathway inhibitor (TFPI) is the natural inhibitor of TF coagulant and signaling activities. It is a Kunitz-type serine proteinase inhibitor that down-regulates tissue factor-initiated blood coagulation. With its Kunitz domains, TFPI exhibits significant homology with human inter-alpha-trypson inhibitor and bovin basic pancreatic trypsin inhibitor. TFPI is the natural inhibitor of TF coagulant and signaling activities. The importance of TFPI in the regulation of blood coagulation is emphasized by how its activity is modulated in human disease. In a factor (F) Xa-dependent feedback system, TFPI binds directly and inhibits the TF-FVII/FVIIa complex. Normally, TFPI exists in plasma both as a full-length molecule and as variably carboxy-terminal truncated forms. TFPI also circulates in complex with plasma lipoproteins. The levels and the dual inhibitor effect of TFPI on FXa and TF-FVII/FVIIa complex offers insight into the mechanisms of various pathological conditions triggered by TF. TFPI may play an important role in modulating TF-induced thrombogenesis and it may also provide a unique therapeutic approach for prophylaxis and/or treatment of various diseases. In addition, studies have shown that TFPI exhibits antiangiogenic and antimetastatic effects in vitro and in vivo. In animal models of experimental metastasis, both circulating and tumor cell-associated TFPI are shown to significantly reduce tumor cell-induced coagulation activation and lung metastasis.